Booster shots available at local pharmacies after completion of initial series
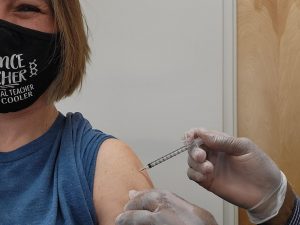
COVID-19 booster shots are available and recommended for adults who are at high risk of contracting the virus or have underlying medical conditions. Booster shots can be administered at local pharmacies such as Tom Thumb, CVS and Walgreens.
October 26, 2021
Booster shots are available to eligible individuals at all locations that also offer COVID-19 vaccinations. Available locations in Coppell include: Tom Thumb, CVS Pharmacy and Walgreens on Denton Tap Road.
“I am all about [everyone] being vaccinated,” Coppell High School pharmacy tech and EMT teacher Gary Beyer said. “When I was a small child, they did mandatory vaccinations for smallpox and polio. I felt fine, and I then and now qualified for a third booster. My thought is that a year, five years, 10 years down the road, you get a flu shot. Every year you’ll get a COVID shot.”
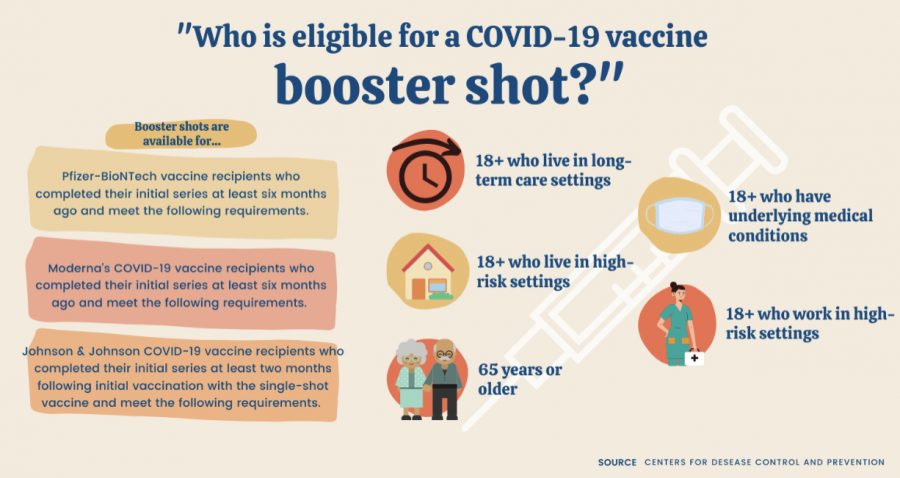
On Oct. 7, the Centers for Disease Control (CDC) recommended a single booster shot of the Pfizer-BioNTech COVID-19 vaccine be administered at least six months after completion of the two-shot preliminary series.
This was the first of the three pharmaceutical COVID vaccine producing companies to advise booster shots following complete vaccination cycles.
On Oct. 14, a Food and Drug Administration (FDA) advisory committee unanimously recommended giving booster shots of Moderna’s COVID-19 vaccine to people ages 65 and older and other vulnerable Americans.
Based on CDC recommendations, third vaccine doses are available now for people who fit the following guidelines: individuals who are 65 or older and 18-plus individuals who live in long-term care settings, have underlying medical conditions, or work or live in high-risk settings.
On Oct. 15, the FDA Vaccines and Related Biological Products Advisory Committee (VRBPAC) unanimously voted 19-0 to recommend Emergency Use Authorization (EUA) for a booster dose of the Johnson & Johnson COVID-19 vaccine for adults aged 18 and older at least two months following initial vaccination with the single-shot vaccine.
CHS students still voice their concerns about safety at school while the vaccine’s long-term efficacy is being questioned.
“The school is still very crowded, and the chance of contracting COVID-19 is still high,” CHS senior Uma Uppuloori said. “I would rather listen to the major pharmaceutical companies, because they know the scientific backgrounds of their products the best. I’d rather take [a booster shot] earlier than later.”
CHS senior Lily Arancheril plans on receiving the booster shot as soon as it is available to her age group. She is opting to receive the booster because her grandparents, who are immunocompromised, live in her home.
“The booster shots were introduced at a good time, [because] they were presented when people were beginning to lose more immunity after being fully vaccinated,” Arancheril said. “I want to be more cautious, especially as people begin to meet that point.”
On Oct. 20, the FDA authorized the use of each of the available vaccines as a heterologous (or “mix-and-match”) booster dose in eligible individuals following completion of primary vaccination with a different available COVID-19 vaccine.
Follow Anette (@AnetteVarghese) and @CHSCampusNews on Twitter.